金属氢化物还原具有反应条件温和,副反应少以及产率高的优点,特别是某些烃基取代的金属化合物,显示了对官能团的高度选择性和较好的立体选择性。在复杂的天然产物的合成中,较之其他还原法显示出更多的优点。最常用的为氢化铝锂(LiAlH4)、硼氢化钾(钠、锂)[K(Na,Li)BH4],以及发展了化学和立体选择性好的试剂,例如硫代硼氢化钠(NaBH2S3)、三仲丁基硼氢化锂LiBH (CH3CH2CH(CH3))3等。
机理
金属复氢化物具有四氢铝离子(AlH4-)或四氢硼离子(BH4-)的复盐结构,这种复合负离子具有亲核性,可向极性不饱和键中带正电的碳原子进攻,继而发生负离子转移而进行还原。There are, however, some differences depending on the reagent and to address those, let’s start with the mechanism of LiAlH4 Reduction:

The hydride addition to the carbonyl is also catalyzed by the lithium ion which serves as a Lewis acid by coordinating to the carbonyl oxygen. This decreases the electron density on the oxygen thus making the C=O bond more susceptible to a nucleophilic attack.
The resulting alkoxide salt can react with the AlH3 and convert it to another source of hydride. However, for simplicity, most often we show only one addition to the carbonyl followed by a protonation of the alkoxide with water or aqueous acidic solutions which gives the final product alcohol.
四氢铝锂是相当强的还原剂。还原酮,醛不在话下,羧酸和酯也能被还原到对应的醇。腈和酰胺也能被还原,得到胺。卤化物和砜也能被还原,它们原位置上变成氢。它也能使能使环氧化合物发生开环反应。反应一般用到本身不与氢化铝锂发生反应同时有良好的溶解性的THF,乙醚等溶剂。氢化铝锂会与水或质子溶剂发生剧烈反应,放出氢气。由于反应剧烈和容易飞散,不可避免发生火灾。取用时要十分的注意。
NaBH4 是另外常用的一种还原剂,对水分稳定,空气中也能使用,最适合工业生产。溶解性是它的一大问题,通常用甲醇或乙醇作溶剂。酯,酰胺,羧酸不能被还原,但是酯的羰基α位若被杂原子取代的情况下是例外(可能是由于相邻的定位作用)。对于α,β-不饱和羰基化合物,优先发生1,4-还原,加入铈盐的情况下则会优先发生1,2-还原(luche还原)。
Sodium borohydride reduces aldehydes and ketones by a similar mechanism with some important differences that we need to mention.
First, NaBH4 is not so reactive and the reaction is usually carried out in protic solvents such as ethanol or methanol. The solvent has two functions here:
1) It serves as the source of a proton (H+) once the reduction is complete
2) The sodium ion is a weaker Lewis acid than the lithium ion and, in this case, the hydrogen bonding between the alcohol and the carbonyl group serves as a catalysis to activate the carbonyl group:

由于这类还原剂的反应活性和稳定性的不同,使用时反应条件也有所不同,氢化铝锂遇水,酸或含羟基,巯基化合物,可分解放出氢而形成相应的铝盐。因而反应需在无水条件下进行,且不能使用带有羟基或巯基的质子溶剂及可被还原的溶剂(DMF, DMSO, CH2Cl2)等。一般用醚类溶剂,主要是无水四氢呋喃或乙醚,其在乙醚中的溶解度为20-30%,四氢呋喃为17%。
氢化硼钾(钠)与上述锂盐有所不同,在常温下,遇水,醇都较稳定,不溶于乙醚及四氢呋喃,能溶于水,甲醇,乙醇而分解甚微,因而常选用醇类作为溶剂。如反应须在较高的温度下进行,则可选用异丙醇,二甲氧基乙醚等作溶剂。在反应液中,加入少量的碱,有促进反应的作用。硼氢化钠比其钾盐更具引湿性,易于潮解,故工业上多采用钾盐。采用氢化硼钾(钠)还原剂反应结束后,可加稀酸分解还原物并使剩余的氢化硼钾生成硼酸,便于分离。
用氢化铝锂还原剂反应结束后,可加入乙醇,含水乙醚或10%氯化铵水溶液以分解未反应的用氢化铝锂和还原物。用含水溶剂分解时,其水量应近于计算量,使生成颗粒状沉淀的偏铝锂而便于分离。如加水过多,则偏铝酸锂进而水解成胶状的氢氧化铝,并与水和有机溶剂形成乳化层,致使分离困难,产物损失较大。
因而,氢硼化物类还原剂,不能在酸性条件下反应,对于含有羧基的化合物的还原,通常应先中和成盐后再反应。
除了常用的 LITHIUM ALUMINIUM HYDRIDE AND SODIUM BOROHYDRIDE, OTHER HYDRIDE REDUCING REAGENTS INCLUDING:
二异丁基氢化铝 (i-Bu)2AlH (Diisobutyl Alminium Hydride; DIBAL)
(Di-isobutyl Aluminum Hydride) is A Bulky For The Partial Reduction Of .
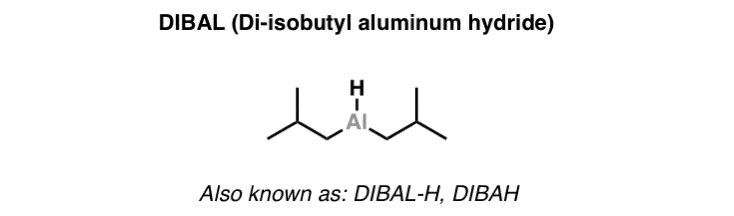 is a strong, bulky . It’s most useful for the reduction of to aldehydes. Unlike , it will not reduce the further if only one equivalent is added. It will also reduce other compounds such as amides, aldehydes, ketones, and .
is a strong, bulky . It’s most useful for the reduction of to aldehydes. Unlike , it will not reduce the further if only one equivalent is added. It will also reduce other compounds such as amides, aldehydes, ketones, and .
Keeping the temperature low (–70°C) tends to keep a lid on the reactivity here. So long as the temperature is kept here for the duration of the experiment and only one equivalent of is added, the is obtained.
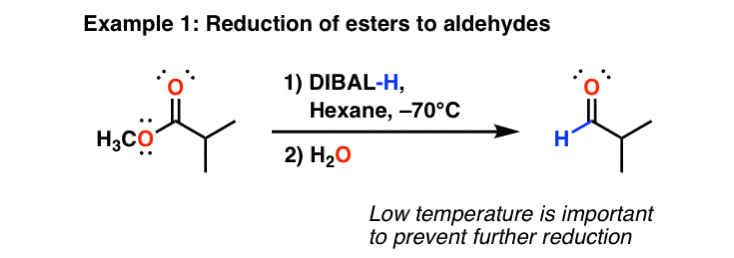
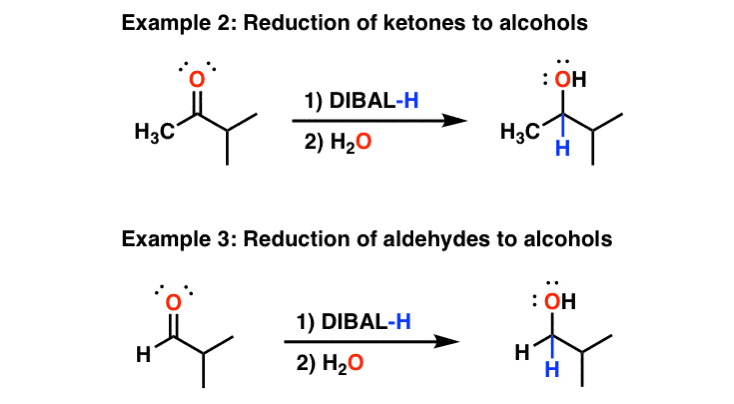 Finally, will also do partial reductions of to imines. The imines are then hydrolyzed to aldehydes upon addition of water. In this respect again differs from LiAlH4, which will reduce all the way to .
Finally, will also do partial reductions of to imines. The imines are then hydrolyzed to aldehydes upon addition of water. In this respect again differs from LiAlH4, which will reduce all the way to .
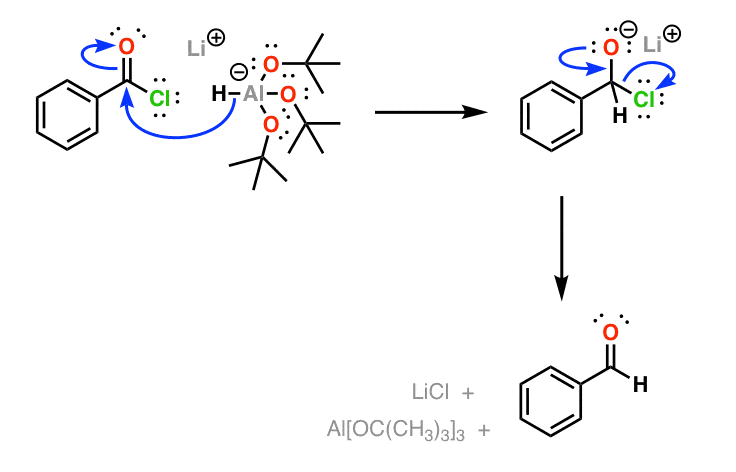
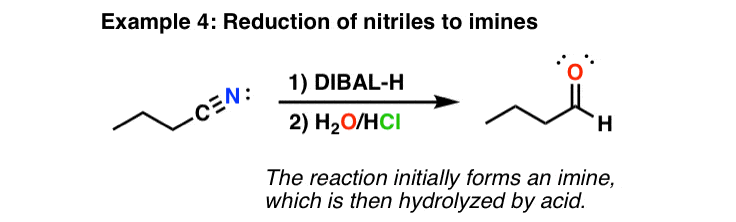 The mechanism for reduction by is a little bit unusual compared to . Whereas is considered a “nucleophilic” reductant – that is, it delivers hydride (H-) directly to a carbon, is an “electrophilic” reductant. That is, the first step in the reaction is coordination of a lone pair from the oxygen (a ) to the aluminum (). It is only after coordinating to its host that delivers its hydride to the carbon, resulting in formation of a neutral intermediate that is stable at low temperatures. Quenching of the reaction then breaks down the , resulting in isolation of the . (“real life” disclaimer: this reaction looks great on paper but can sometimes be difficult to achieve in practice).
The mechanism for reduction by is a little bit unusual compared to . Whereas is considered a “nucleophilic” reductant – that is, it delivers hydride (H-) directly to a carbon, is an “electrophilic” reductant. That is, the first step in the reaction is coordination of a lone pair from the oxygen (a ) to the aluminum (). It is only after coordinating to its host that delivers its hydride to the carbon, resulting in formation of a neutral intermediate that is stable at low temperatures. Quenching of the reaction then breaks down the , resulting in isolation of the . (“real life” disclaimer: this reaction looks great on paper but can sometimes be difficult to achieve in practice).
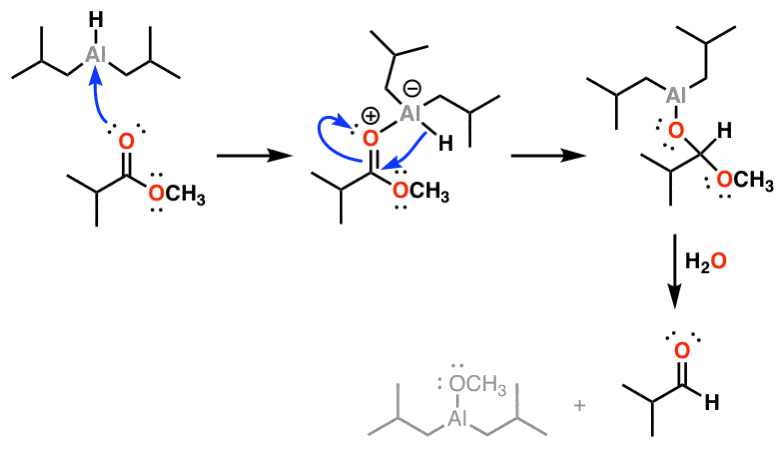
The same mechanism is in effect in the reduction of to imines (and then on to aldehydes). Coordination of the nitrogen to aluminum is followed by delivery of hydride, and from there, addition of water leads to hydrolysis of the imine and subsequent isolation of the .
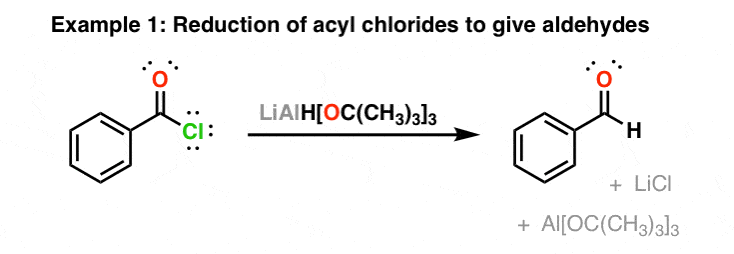
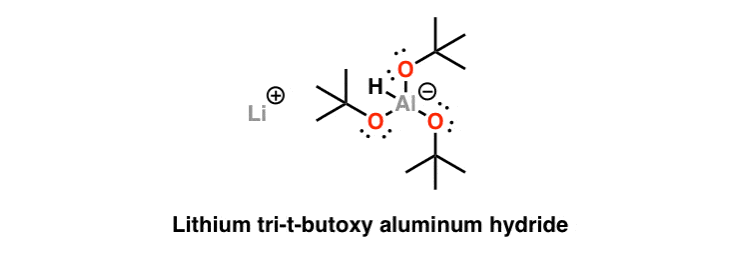
Lithium Tri-tert-butoxyaluminum Hydride (LiAlH(Ot-Bu))
Reductive amination by NaBH3CN is sometimes done in the presence of certain Lewis acids (ex. Ti(OiPr)4, TiCl4, or ZnCl2). The aldehyde or ketone is typically prestirred with the amine in the presence of the Lewis acid, then treated with NaBH3CN. This procedure is useful in cases where imine formation is poor, when the use of excess amine isn't possible, or acidic conditions aren't well tolerated.
NaBH3CN can effectively be used in H2O and protic solvents (ex. MeOH or EtOH), unlike sodium triacetoxyborohydride (STAB) which is quickly degraded by H2O and protic solvents.
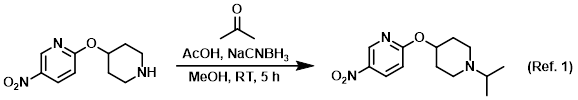
To a solution of 10% AcOH in MeOH was added the SM (1 equiv) and dry acetone (5 equiv). The solution was stirred at RT 1 h, after which time it was cooled to 0 C and treated with NaCNBH3 (1.5 equiv). The reaction was stirred at RT for 5 h. The mixture was concentrated and the residue brought to pH = 10 using Na2CO3. The mixture was extracted with EtOAc and the org layer was washed with H2O, brine, dried (Na2SO4), and concentrated to dryness. The crude material was purified by silica gel column chromatography (2% MeOH/DCM) to provide the pdt as a yellow solid. (ref. 1 WO2007084786, page 112).
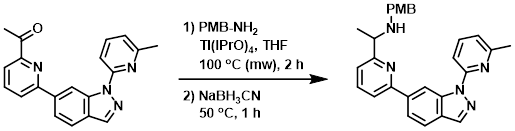
A mixture of the SM (260 mg, 0.79 mmol), PMB-NH2 (162 mg, 1.18 mmol), and Ti(iPrO)4 (168 mg, 1.58 mmol) in THF (10 mL) was stirred in a microwave reactor at 100 C for 2 h. After cooling to RT, NaBH3CN (98 mg, 1.58 mmol) was added to the mixture. The reaction mixture was stirred at 50 C for 1 h. The mixture was poured into H2O (10 mL) and extracted with EtOAc (20 mL). The org phase was concentrated and the residue was purified by silica gel column chromatography (6:1 PE/EtOAc) to provide the product as a yellow solid. [180 mg, 50%] [Patent Reference: WO2016011390, page 104]
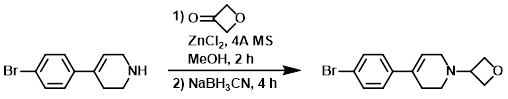
To a solution of the SM (1.10 g, 4.61 mmol) in MeOH (15 mL) was successively added oxetan-3-one (1.66 g, 23.01 mmol), 4A molecular sieves (0.5 g), and ZnCl2 (3.14 g, 23.01 mmol). After stirring 2 h, the reaction mixture was treated with NaBH3CN at 0 C, and stirring was continued for another 4 h. The mixture was diluted with H2O (20 mL), EtOAc (25 mL), and sat aq NaHCO3 (10 mL). The layers were separated and the aq layer was further extracted with EtOAc (3 x 25 mL). The combined organics were washed with brine (50 mL), dried (Na2SO4), and concentrated. The residue was purified by silica gel column chromatography (50% EtOAc/hexane) to provide the product as a colorless syrup. [1.1 g, 81%][Patent Reference: WO2015088045, page 108]
Lithium borohydride
Lithium borohydride (LiBH4) is a borohydride and known in organic synthesis as a reducing agent for esters. Although less common than the related sodium borohydride, the lithium salt offers some advantages, being a stronger reducing agent and highly soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride.

LiBHEt3(Lithium Triethylborohydride: Super-Hydride)

Lithium triethylborohydride (LiEt3BH), commonly abbreviated to LiTEBH or Superhydride, is a powerful and selective reducing agent used in inorganic and organic chemistry. The structure of LiTEBH causes the compound to be a very strong hydride source. Hydrogen is more electronegative than boron which causes the B-H bond to be strongly polarized with boron having a partial positive charge and hydrogen having a partial negative charge. The ethyl groups on the boron also aids to this abnormal polarizability by removing additional electron density from the boron making it even more electropositive. This polarization of the B-Et and B-H bonds causes the hydrogen to be in the (-I) oxidation state instead of its usual (+I) oxidation state which leads to its high reactivity with atoms that can accept electrons to allow the hydrogen to go to its (+I) oxidation state.LiTEBH is far more powerful than lithium borohydride and more powerful than lithium aluminum hydride (LAH) in many cases. One of the main advantages of LiTEBH is that it is safer than LAH.
LiTEBH rapidly reduces:
1. Aldehydes, ketones, acid chlorides and esters to alcohols
2. Lactones to diols
3. Acid anhydrides to alcohols
4. α,β-enones by 1,4-addition to give lithium enolates
5. Disulfides to thiols
6. Tertiary amides to an alcohol
LiTEBH reacts violently and exothermically with water, alcohols, or acids releasing flammable hydrogen gas which can ignite explosively and the pyrophoric triethylborane vapor can ignite spontaneously. It can cause severe eye, skin, and respiratory tract burns.
Sodium bis(2-methoxyethoxy)aluminium hydride
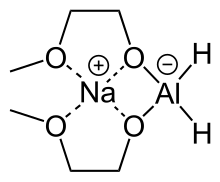
Sodium bis(2-methoxyethoxy)aluminium hydride ( trade names Red-Al) is a complex hydride reductant with the formula NaAlH2(OCH2CH2OCH3)2. The trade name Red-Al refers to its being a reducing aluminium compound. It is used predominantly as a reducing agent in organic synthesis. The compound features a tetrahedral aluminium center attached to two hydride and two alkoxide groups, the latter derived from 2-methoxyethanol. Commercial solutions are colorless/pale yellow and viscous. At low temperatures (<-60°C), the solution solidifies to a glassy pulverizable substance with no sharp melting point.
RED-AL is a versatile hydride reducing agent. It readily converts epoxides, aldehydes, ketones, carboxylic acids, esters, acyl halides, and anhydrides to the corresponding alcohols. Nitrogen derivates such as amides, nitriles, imines, and most other organonitrogen compounds are reduced to the corresponding amines. Nitroarenes can be converted to azoxyarenes, azoarenes, or hydroazoarenes, depending on the reaction conditions.
Some common functional group reductions using SMEAH can be found below:

As a reagent, RED-AL is comparable with lithium aluminium hydride (LAH, LiAlH4).
It is a safer alternative to LAH and related hydrides. RED-AL is exhibits similar reducing effects, but does not have the inconvenient pyrophoric nature, short shelf-life, or limited solubility of LAH. Upon contact with air and moisture, it reacts exothermically but does not ignite, and tolerates temperatures up to 200 °C. Under dry conditions it has unlimited shelf life. It is soluble in aromatic solvents, whereas LAH is only soluble in ethers. For example, a solution greater than 70 wt.% concentration in toluene is commercially available. The reagent can be modified to effect partial reductions.[1]
RED-AL in toluene under reflux has been used to reduce aliphatic p-toluenesulfonamides (TsNR2) to the corresponding free amines and is one of the few reagents that can carry out this challenging reduction in general settings. Notably, LiAlH4 does not reduce this functional group unless forcing conditions are used.
Sodium triacetoxyborohydride
Sodium triacetoxyborohydride, also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a chemical compound with the formula Na(CH3COO)3BH. Like other borohydrides, it is used as a reducing agent in organic synthesis.
Sodium triacetoxyborohydride is a milder reducing agent than sodium borohydride or even sodium cyanoborohydride. It reduces aldehydes but not most ketones. It is especially suitable for reductive aminations of aldehydes and ketones.
However, unlike sodium cyanoborohydride, the triacetoxyborohydride hydrolyzes readily, nor is it compatible with methanol. It reacts only slowly with ethanol and isopropanol and can be used with these.
This colourless salt is prepared by protonolysis of sodium borohydride with acetic acid:
NaBH4 + 3 HO2CCH3 → NaBH(O2CCH3)3 + 3 H2
Zn(BH4)2(Zinc Borohydride)
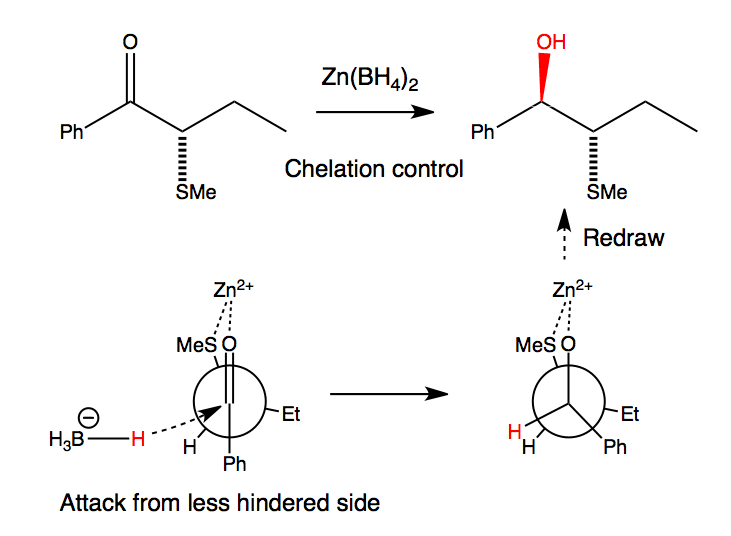
利用锌的螯合能,能发生syn-非对映选择性还原
Zinc can chelate to the two heteroatoms with lone pairs, sulfur and the carbonyl group, and here it changes the conformation of the starting material. No longer does the most reactive or most populated conformation place the electronegative S atom perpendicular to the C=O; instead it prefers SR to lie as close to the carbonyl oxygen as possible so that Zn can bridge between S and O. Attack of borohydride from the less hindered side (next to H rather than Et) leads to the anti diastereoisomer.
? ?
?
Lithium Tri(sec-butyl)borohydride; L-Selectride)
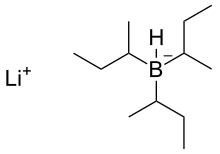
L-selectride is an organoborane. It is used in organic chemistry as a reducing agent, for example in the reduction of a ketone, as part of Overman's synthesis of strychnine.

Under certain conditions, L-selectride can selectively reduce enones by conjugate addition of hydride, owing to the greater steric hindrance the bulky hydride reagent experiences at the carbonyl carbon relative to the (also-electrophilic) β-position. L-Selectride can also stereoselectively reduce carbonyl groups in a 1,2-fashion, again due to the steric nature of the hydride reagent.
N-selectride and K-selectride are related compounds, but instead of lithium as cation they have sodium and potassium cations respectively. These reagents can sometimes be used as alternatives to, for instance, sodium amalgam reductions in inorganic chemistry.